Today’s steel production process relies on fossil fuels, so the steel industry is one of the largest greenhouse gas emitters.
Therefore, the steel industry is strongly demanding to reduce CO2 emissions. As a specific goal, the International Energy Agency (IEA) set a sustainable development scenario (SDS: Sustainable Development Scenario) in 2020. The goal of this development scenario is to achieve zero CO2 emissions related to global energy use in 2070 in accordance with the Paris Agreement, and the steel industry to reduce emissions by 54% in 2050 compared to 2019; in 2070, emissions need to be reduced by 90%. The COP26 “Glasgow Climate Agreement” adopted last year clearly stated the goal of controlling the increase in global temperature within 1.5°C by the end of this century, requiring the steel industry to further accelerate its emission reduction rate than the above scenario.
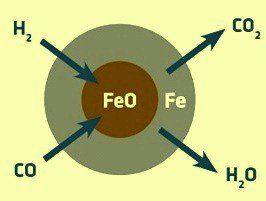
There are two main components of the MIDREX method. One is a shaft furnace for reducing iron ore raw materials (pellets or lumps) to reduced iron, and the other is a reforming furnace for producing reducing gas required for shaft furnace reduction. (reforming furnace). The feature of this process is that the CO2 in the top gas is used in the reformer to reform natural gas into hydrogen (H2) and carbon monoxide (CO) to produce reducing gas (about 55% of H2 and about 36% of CO). Because of the use of hydrogen-rich reducing gas and the effective use of CO2 in the furnace top exhaust gas, the CO2 emission is 20%-40% lower than that of the blast furnace method using coke. In addition, part of the hydrogen can be added to the natural gas, even without major modification of the equipment, 100% of the natural gas can be replaced with hydrogen, thereby greatly reducing CO2 emissions.
The reduced iron (DRI) produced by the MIDREX process shaft furnace includes normal temperature reduced iron (CDRI), thermal reduced iron (HDRI) and hot briquetted iron (HBI), which can be manufactured in various combinations according to the application.
After reduction, CDRI that has been cooled to normal temperature has most of the pores left empty due to the removal of oxygen, and it has the property of being oxidized again when it is exposed to air or the like during long-term storage. Therefore, the high-temperature reduced iron shortly after leaving the shaft furnace is compressed into a block between two rollers to reduce the void ratio is HBI. Therefore, HBI has good reoxidation resistance, which not only solves the problems of long-term storage and sea transportation, but also prevents the reduction of utilization rate due to dust generation during the treatment process. Since HBI is the preferred form of foreign sales, a supply chain is formed, and HBI manufactured in places with low production costs and convenient transportation can be bought in the market.
On the other hand, when DRI is used in the electric furnace factory adjacent to the direct reduction plant, the reduced DRI above 600°C is used directly in the electric furnace at high temperature, which can reduce a large amount of melting energy, which helps to improve the productivity of the electric furnace and reduce CO2 emissions.
In the global carbon-neutral campaign, the trend of using steel scrap as the main raw material to reduce CO2 emissions is expanding. However, due to the limited production of scrap steel, the demand for reduced iron will be greatly expanded as a substitute or supplementary electric furnace raw material for scrap steel.
In addition, technology that can reduce CO2 emissions from blast furnaces by 20% by charging reduced iron (HBI) into blast furnaces has also been demonstrated. As a means of achieving immediate reduction of CO2 emissions using existing blast furnaces, it is expected to expand the use of reduced iron in blast furnaces.
In the direct reduction-electric furnace method, high-grade iron ore with less gangue components such as SiO2 and Al2O3 is used. This is because the gangue component in iron ore will form slag when it is melted in an electric furnace, and when oxidation refining is carried out in an electric furnace, in order to prevent the iron content from being oxidized and lost to the slag, it is desirable to have a small amount of slag, that is, a small amount of gangue . However, the supply of high-grade iron ore is limited, and it is a big problem to use low-grade iron ore, which is currently used in the blast furnace method and has an abundant supply, in the direct reduction-electric furnace method. For this problem, we are developing a solution to manufacture pig iron from reduced iron by introducing a reduced iron electric furnace. This is to melt the reduced iron with more gangue components into the electric furnace with a reduced iron electric melter to remove the gangue components, and at the same time suppress the loss of iron content into the slag under a reducing atmosphere, thereby realizing low-grade iron ore usage of.
In the MIDREX plant using natural gas, if electricity with low CO2 emissions can be used, hydrogen production will be carried out, and natural gas will be partially replaced with hydrogen, eventually achieving 100% hydrogen substitution. In addition, if it is a condition where CO2 can be stored, CO2 can also be separated and recovered by installing CO2 removal equipment at the MIDREX plant.
In this way, with the MIDREX process as the center, not only can it solve short-term problems, but it can also draw a realistic path for future carbon-free iron production from a long-term perspective. Facing such a major issue as carbon neutrality in the steel production process, we will contribute by developing and expanding the application of the MIDREX process.